Hackaday
1M
378
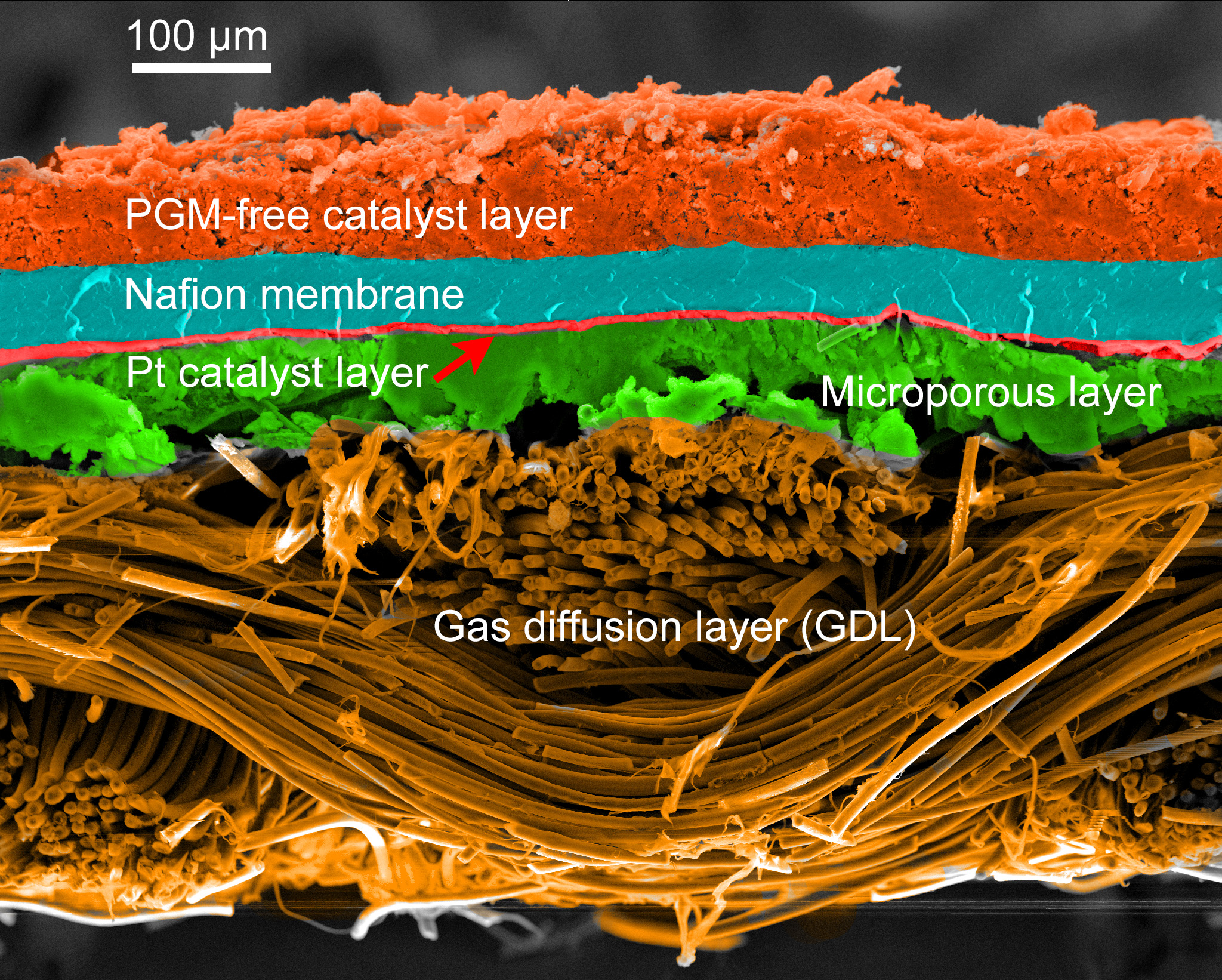
Image Credit: Hackaday
A Brief History of Fuel Cells
- Fuel cells are devices that convert a chemical reaction into electricity, similar to batteries but with key differences.
- They consume chemicals and produce electricity as long as there is fuel, while batteries store energy.
- Fuel cells have an anode, cathode, and electrolyte, where a catalyst causes fuel to oxidize and generate electric current.
- The first fuel cell was made by Sir William Grove in 1838, using dilute acid and copper sulphate.
- Different types of fuel cells use varied fuels and produce byproducts like water, carbon dioxide, and electricity.
- Common types include alkaline, solid acid, phosphoric acid, molten carbonate, and solid oxide fuel cells.
- Fuel cells have been widely used in space missions due to their efficiency and ability to utilize hydrogen and oxygen.
- Stationary and mobile fuel cells offer benefits like no moving parts and clean energy production for various applications.
- Efforts have been made to introduce fuel cells in vehicles like buses, trains, forklifts, airplanes, and motorcycles.
- Although DIY fuel cell projects exist, commercial adoption faces challenges like hydrogen storage and distribution.
Read Full Article
22 Likes
For uninterrupted reading, download the app