Bioengineer
1w
92
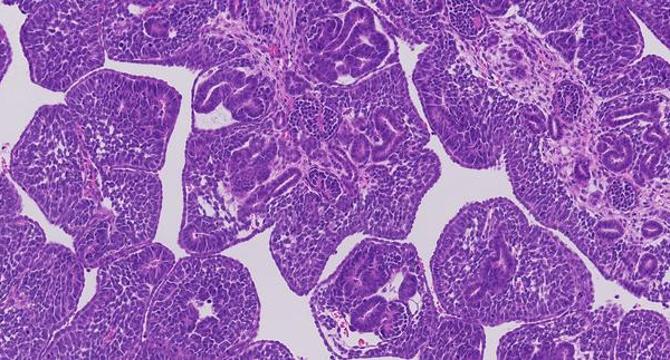
Image Credit: Bioengineer
Childhood Kidney Cancer Exhibits Millions of Genetic Mutations, Paving the Way for New Treatment Opportunities
- A groundbreaking study has revealed that some childhood kidney tumors exhibit an unexpectedly high number of DNA alterations, challenging previous notions about genetic simplicity in pediatric cancers.
- By integrating innovative genomic sequencing techniques, researchers discovered that each cancer cell in Wilms tumors harbored 72 to 111 unique genetic changes beyond bulk sequencing findings, amounting to millions of mutations per tumor.
- The increased mutational burden in pediatric tumors suggests potential for rapid evolution and adaptability, impacting responses to conventional therapies and immunotherapies.
- Identification of specific mutations like a FOXR2 gene alteration in Wilms tumor subtypes presents opportunities for tailored diagnostics and individualized treatment approaches.
- The study's precision at the single-cell level through nanorate sequencing and organoid analysis unveils intricate genetic landscapes, reshaping diagnostic and therapeutic strategies for childhood cancers.
- The findings challenge the notion of genetic simplicity in childhood tumors, offering new possibilities for repurposing adult cancer therapies for pediatric patients and enhancing treatment accessibility.
- Collaborations between genomic researchers and clinical oncologists play a critical role in leveraging advanced sequencing technologies for personalized and effective pediatric cancer treatments.
- This research marks a paradigm shift in pediatric oncology, highlighting the complex genetic nature of childhood cancers and the potential for advanced therapeutic interventions to improve outcomes.
- The interdisciplinary approach showcased in this study underscores the importance of integrating technical innovation with clinical insights to drive advancements in cancer research and patient care.
- The study's implications extend beyond Wilms tumor, offering insights into cancer heterogeneity, evolution, and potential treatment strategies that could benefit pediatric cancer patients more broadly.
Read Full Article
5 Likes
For uninterrupted reading, download the app