Bioengineer
3d
127
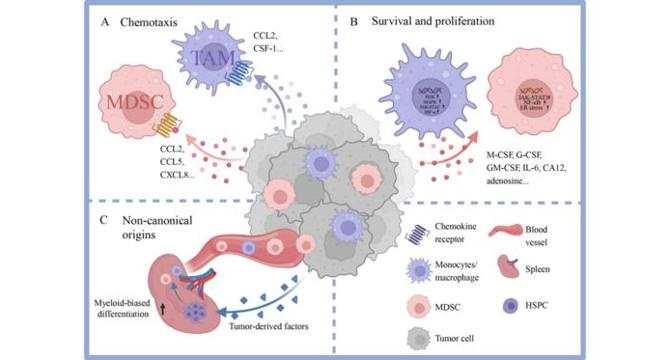
Image Credit: Bioengineer
Myeloid Cells: Central Architects of the Tumor Microenvironment
- Myeloid cells, including TAMs and MDSCs, play crucial roles in the tumor microenvironment, influencing cancer progression and therapeutic resistance through their plasticity and diverse functions.
- TAMs and MDSCs are derived from monocytes and bone marrow progenitors, with contributions from extramedullary hematopoiesis driven by tumor-secreted factors.
- Metabolic reprogramming is essential for myeloid cell functions in the TME, with TAMs utilizing glycolysis, lactate production, and lipid accumulation to modulate immune responses.
- MDSCs impair T cell function through amino acid metabolism, while relying on glutamine and FAO pathways for energy and survival.
- Therapeutic approaches target myeloid cell recruitment, survival, and metabolic dependencies to mitigate their pro-tumoral effects.
- Advancements in single-cell transcriptomics reveal myeloid heterogeneity, with subsets exhibiting distinct roles and responses to therapy based on tumor type and microenvironment.
- Interactions between myeloid cells and other immune/stromal constituents shape complex ecosystems that influence tumor fate and therapeutic responses.
- Reprogramming myeloid function through metabolic interventions and drug repurposing offers promising avenues to enhance anti-tumor immunity.
- Precision immunomodulation targeting myeloid subsets can transform the therapeutic landscape by reversing immune suppression and improving T cell efficacy.
- Understanding myeloid cell biology in oncology paves the way for personalized immunotherapy, emphasizing their pivotal role in fighting cancer.
Read Full Article
7 Likes
For uninterrupted reading, download the app