Bioengineer
5d
260
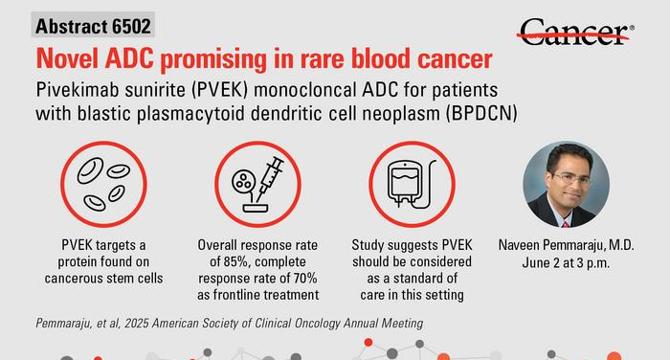
Image Credit: Bioengineer
New Antibody-Drug Conjugate Demonstrates Promising Safety and Efficacy in Rare Blood Cancer, ASCO Reports
- A novel antibody-drug conjugate named pivekimab sunirine (PVEK) has shown promising results in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive blood cancer.
- PVEK targets the CD123 receptor highly expressed on BPDCN cells, delivering a potent cytotoxic agent to malignant cells while minimizing systemic toxicity.
- Clinical trial data presented at the 2025 ASCO Annual Meeting revealed an overall response rate of 85% and a 70% complete remission rate in newly diagnosed BPDCN patients receiving PVEK.
- The median overall survival for this cohort was 16.6 months, indicating PVEK's potential to improve patient outcomes significantly.
- PVEK's safety profile was favorable, with peripheral edema being the most commonly observed adverse event, manageable in the patient population.
- Compared to the current standard of care, tagraxofusp-erzs, PVEK offers a distinct toxicity profile, potentially providing a therapeutic advantage.
- The antibody component of PVEK recognizes the CD123 antigen, leading to targeted cytotoxicity in BPDCN tumor cells while sparing non-malignant cells.
- The CADENZA trial involving 84 participants demonstrated PVEK's efficacy in both treatment-naïve and relapsed/refractory BPDCN cohorts, with ongoing analyses underway.
- The success of PVEK aligns with the precision medicine trend in oncology, offering targeted cytotoxic delivery that addresses challenges like heterogeneity and drug resistance.
- PVEK's development showcases collaborative efforts between academic research and pharmaceutical innovation, emphasizing the importance of specialized cancer centers in advancing translational research.
- The emergence of PVEK as a potent antibody-drug conjugate represents a significant advancement in BPDCN management, promising improved therapeutic outcomes through targeted therapy.
Read Full Article
15 Likes
For uninterrupted reading, download the app