Physicsworld
1M
192
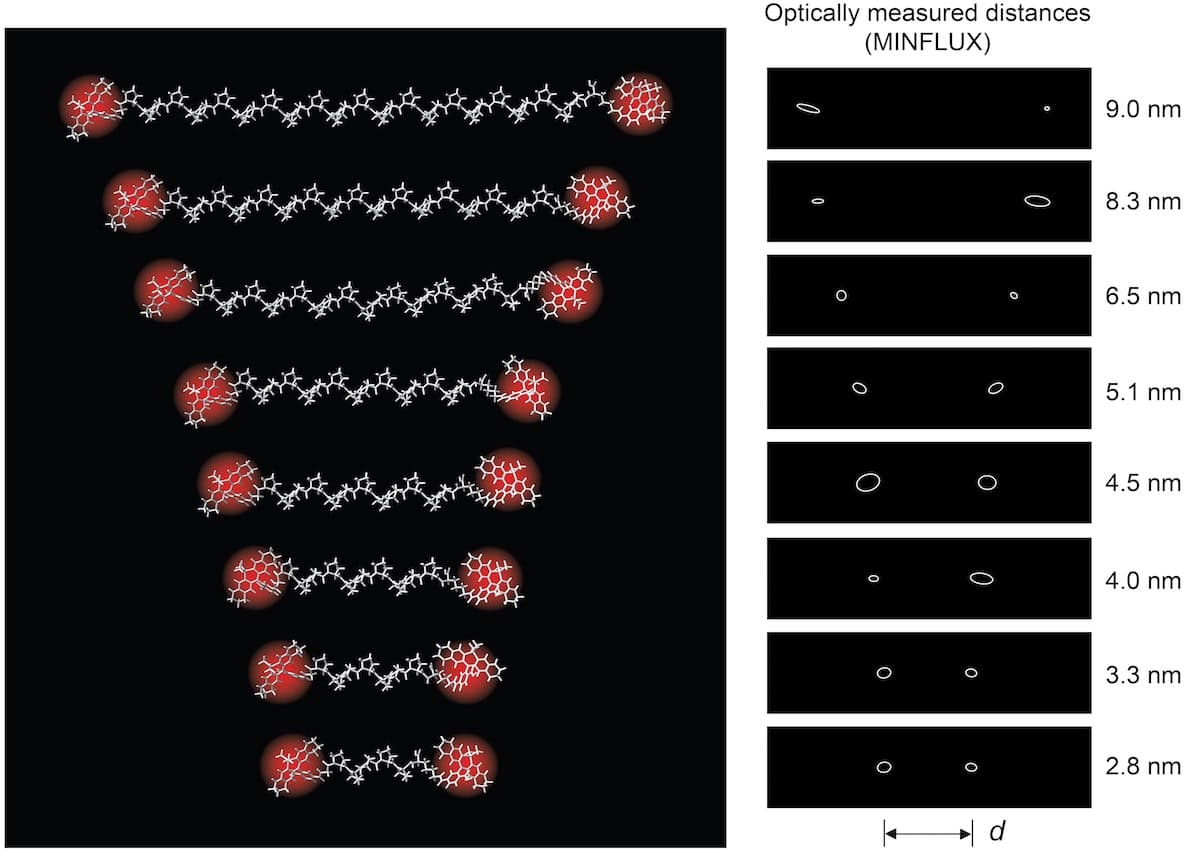
Image Credit: Physicsworld
Optical technique measures intramolecular distances with angstrom precision
- Physicists in Germany have used a MINFLUX enhanced version of an optical fluorescence microscopy technique to measure intramolecular distances smaller than 10 nm precisely. The technique has 1-Angstrom precision and can be used to study biological processes such as protein interactions. Conventional microscopy cannot distinguish between two features of an object separated by less than half the wavelength of the light used to image them due to diffraction. MINFLUX overcomes diffraction by exciting individual fluorescent groups and leaving others dark. A new type of fluorescent dye molecule that can be switched in succession using UV light but not interact with each other was introduced to measure positions with single fluorescent molecules to within 0.1 nm. The technique demonstrated precision of 0.1 nm while measuring distances of 1–10 nanometres in polypeptides and proteins. MINFLUX can serve as a reliable alternative for monitoring sub-10-nm distances.
- MINFLUX first turns on individual molecules and then determines their position by scanning a beam of light across them with a doughnut-shaped intensity profile.
- Localization process involves relating the unknown position of the fluorophore to the known position of the centre of intensively light intense beam. The technique reduces the required levels of detected photons by a factor of 100 compared to traditional camera-based techniques, leading to 10-fold precision increase.
- One of the crucial prerequisites for the development of MINFLUX was the sequential ON/OFF switching of the fluorophores emitting fluorescence. The fluorescent molecules should have independent behavior in the silent (OFF-state) and emitting (ON-state) states.
- The technique is exciting as it brings access to conformation details of biological molecules and can quantify measurements even within them. Researchers are looking to establish MINFLUX as a standard tool for quantifying the mechanics of proteins.
Read Full Article
11 Likes
For uninterrupted reading, download the app