Medium
4w
382
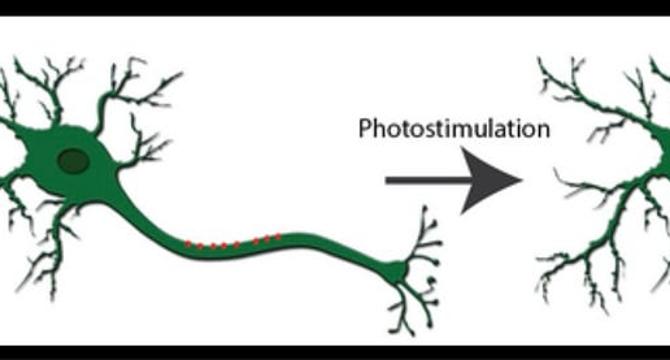
Image Credit: Medium
Optogenetics: Illuminating Neural Circuits with Light
- Optogenetics integrates genetic engineering and optics to control and monitor specific cells, like neurons, using light.
- Researchers introduce genes encoding photosensitive proteins (opsins) into cells, allowing control with high temporal and spatial resolution.
- Karl Deisseroth pioneered optogenetics in the mid-2000s, enabling precise neural circuit control with Channelrhodopsin-2.
- The technique combines genetics and optics for on-demand control of excitable cells, modulating neural excitability with light.
- Light-sensitive proteins like Channelrhodopsin-2 or Halorhodopsin are commonly used to either excite or suppress neural activity.
- Genetic delivery methods like viral vectors or transgenic animal lines are employed to express opsins in targeted cells.
- Light delivery methods include optical fibers or miniaturized LEDs/lasers implanted to illuminate specific brain regions in living animals.
- Optogenetics has revolutionized neuroscience by allowing precise control over neuronal firing, mapping neural circuits, and linking brain activity to behavior.
- Potential medical applications include vision restoration, neurological and psychiatric disorder treatments, and cardiac arrhythmia control using optogenetic therapies.
- Recent advancements in optogenetics include engineered opsins for improved features, advanced light delivery systems, and wireless multi-site stimulation technologies.
Read Full Article
23 Likes
For uninterrupted reading, download the app